‘Doughnut’ beams help physicists see incredibly small objects
In a new study, researchers at CU Boulder have used doughnut-shaped beams of light to take detailed images of objects too tiny to view with traditional microscopes.
The new technique could help scientists improve the inner workings of a range of “nanoelectronics,” including the miniature semiconductors in computer chips. The discovery was highlighted Dec. 1 in a special issue of Optics & Photonics News called Optics in 2023.
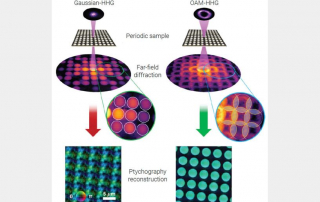
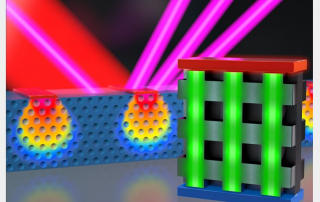
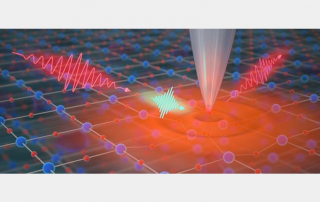
The research is the latest advance in the field of ptychography, a difficult to pronounce (the “p” is silent) but powerful technique for viewing very small things. Unlike traditional microscopes, ptychography tools don’t directly view small objects. Instead, they shine lasers at a target, then measure how the light scatters away—a bit like the microscopic equivalent of making shadow puppets on a wall.
So far, the approach has worked remarkably well, with one major exception, said study senior author and Distinguished Professor of physics Margaret Murnane.
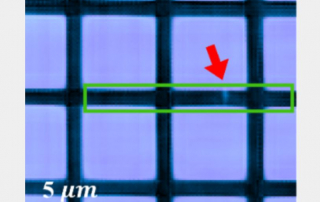
“Until recently, it has completely failed for highly periodic samples, or objects with a regularly repeating pattern,” said Murnane, fellow at JILA, a joint research institute of CU Boulder and the National Institute of Standards and Technology (NIST). “It’s a problem because that includes a lot of nanoelectronics.”